Battery Basics: An Environmentally Friendly Shift
If you’re reading this blog post, there’s a good chance you have lived in a home where a pack of AA batteries was always on standby, and you used them to power your digital camera and Nintendo Game Boy devices. And there was probably a time when you or your parents bought those little round coin cell batteries to power wristwatches. Today, batteries are still all around us. We use them in our remote-controlled toys, smoke detectors, laptops, tablets, and smartphones. There are also new emerging applications for batteries based on market trends such as safe disposal. Continuous glucose monitor (CGM) patches, smart labels for package shipping, and payment cards with changing security PIN displays are all examples of new battery applications where safe disposal is a top priority.
Primary vs. Secondary Cell Batteries
Why the trip down memory lane and into the future? If you can relate to any of the memories or emerging applications above, then you already know something about the two most common categories of batteries: primary and secondary. Primary cell batteries are non-rechargeable batteries. The AA batteries used in your old digital camera or the CR2032 coin cell used in your dad’s watch are examples of primary cell batteries. In contrast, the likely Lithium Ion (Li-ion) battery in your smartphone is rechargeable. Batteries that are rechargeable are known as secondary cell batteries. In summary:
- Primary cell batteries: non-rechargeable; examples include alkaline and lithium batteries
- Secondary cell batteries: rechargeable; examples include Lithium ion (Li-ion), Nickel-cadmium (NiCd), Nickel-metal hydride (NiMH), etc. batteries
Both battery types pack a lot of power for their small size but have their power and environmental drawbacks. For example, primary cell lithium batteries historically have had a higher energy density than their rechargeable Lithium Ion counterparts but need to be disposed of after one use (Sciencing). And although a secondary cell battery’s rechargeable status may seem better for the environment initially, after being recharged many times they too will become ineffective and need to be thrown away.
Disposable Thin-Film Batteries: A Sustainable Alternative
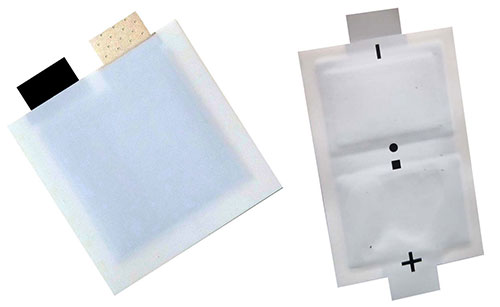 (Image source: Molex)
(Image source: Molex)
There are many different subcategories and types of batteries, but one corner of the battery world getting increased attention from the growing Internet of Things (IoT), wearables, and environmental sensor markets is flexible, printed, thin-film batteries (IDTechEx). For simplicity, we will focus our attention on the general category of thin-film, flexible, printed batteries known as solid-state thin-film batteries. This name gives us a little bit more insight into the type of battery being discussed. Solid-state batteries are just that — solid — with no gels or liquids inside their structure (Qnovo). Thin-film batteries are designed and manufactured with very thin layers or films of materials, and their thin design is part of what makes them so flexible and attractive to the wearable sensing market. Many of these solid-state thin-film batteries meet market needs for thinness and flexibility, but they are often still designed with Lithium-based chemistries or other chemistries that make them potentially toxic to the environment.
The widespread use and toxicity of certain batteries becomes problematic when we consider the vast quantity of batteries thrown away every year. As demand for electronic devices such as laptops and smartphones has increased, so has their contribution to the amount of waste generated each year (Sciencing). Batteries are generally not biodegradable and when carelessly thrown away can run the risk of releasing toxic metals and chemicals into the ground. Many countries now have regulations around battery disposal and offer recycling programs. These programs help recycle the metal from batteries and can help curb the negative impacts of battery disposal on the environment.
Battery disposal regulations, coupled with the increasing need to power and connect more devices to the Internet of Things, are motivating companies to explore alternatives to hazardous battery chemistries that are safe and sustainable. Molex’s new line of thin-film batteries is one such solution. Unlike their Lithium battery cousins, the batteries are designed with a Zinc Manganese Dioxide chemistry and are safer and more convenient for the end user to dispose.
The Molex Advantage
The Internet of Things has helped expand wireless transmission applications such as wearable sensing devices and powered smart labels. However, many printed batteries cannot provide the peak current required for wireless data transmission. Molex meets this challenge by offering thin film batteries that do meet these peak current requirements. Additionally, our batteries are low-profile, flexible, and safer to dispose than lithium-based chemistries. Molex’s thin film batteries have several unique features and advantages. We license a unique technology that allows us to manufacture the anode and cathode on the same plane. This vertical construction allows for reduced internal resistance and footprint. A critique of lithium-based power sources is the danger of self-ignition when not handled carefully. In contrast, Molex thin film batteries are stable in normal storage and operating conditions. They are considered non-hazardous and can be disposed of in normal municipal waste. These batteries also have higher peak current and usable capacity than batteries with similar chemistries, which is important for devices wirelessly sensing or transmitting data. The batteries’ thin and flexible form factor allows them to be bent over curved surfaces to fit a wide variety of products and increase design flexibility. Finally, the batteries are available in a 1.5V and 3V configuration, and the features above make them ideal for low-power, single-use applications.
Putting Thin-Film Batteries to Work
Real-life use cases help highlight applications where features such as a low-profile, flexibility, disposability, and small footprint are highly valued and where we can expect the thin-film battery market to continue growing. One particularly interesting use case is utilizing thin-film batteries in ultra-high frequency (UHF) smart temperature tags. The tags are about the size of a credit card and a little thicker than standard printer paper. They are used by cold chain logistics managers for temperature-sensitive products. These smart temperature tags utilize a combination of technologies including RFID, intelligent temperature sensing, and printed thin-film batteries to accurately track time and temperature during product transportation and storage (Enfucell).
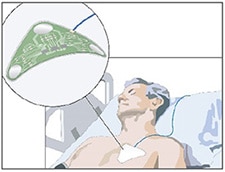
Additionally, the consumer, cosmetics and medical markets are experimenting with applications for thin-film batteries. At the crossroads of the consumer and cosmetics markets is an electrified eye-mask application. The mask features a microcurrent device composed of the flexible printed battery, electrodes, adhesive tape and a covering sheet (Enfucell). Placement of the patch on the skin instantaneously creates a current loop, and the cosmetic flows from the active electrodes in the mask to the skin (Enfucell). Other consumer market applications of thin-film batteries are in wearable electronic and sports monitoring devices, including a low-energy Bluetooth (BLE) sensor patch that gets attached to the side of a golf club head to measure acceleration and angular velocity (Enfucell). Medical applications for disposable thin-film batteries include patient diagnostic, therapeutic, and monitoring devices.
In the last 200 years, great strides have been made in the development of new and different types of batteries to satisfy a world increasingly hungry to power the many devices and applications we use every day. More recently companies have begun to develop batteries made with materials that are abundant, sustainable, and safe for both the environment and people. Printed, flexible, thin-film batteries with organic chemistries such as zinc magnesium dioxide (highlighted in this video) and related chemistries are evidence of a new more environmentally friendly future for batteries. Markets such as industrial, Internet of Things, consumer, and medical have already been successfully experimenting and manufacturing product powered by thin-film batteries. More development is needed to increase the capacity and manufacturability of these batteries, but one pressing question continues to drive developers: where can we use thin-film batteries next?

Have questions or comments? Continue the conversation on TechForum, DigiKey's online community and technical resource.
Visit TechForum